Industry Leader in Scientific Cloud Platform Solutions
Whether you are responsible for global operations, coordinate multiple sites or manage an internal team, our cloud based architecture can accommodate any variety of workflows and organizational structures.
The GoLIMS Platform has been successfully implemented and adopted in a variety of industry segments, including: Animal Health and Nutrition, Contract Research Organizations, Crop Science, Biofuels, and Research & Development Departments.


GoLIMS Industries Served
Agricultural Science
Contract Research Organizations
Key Features
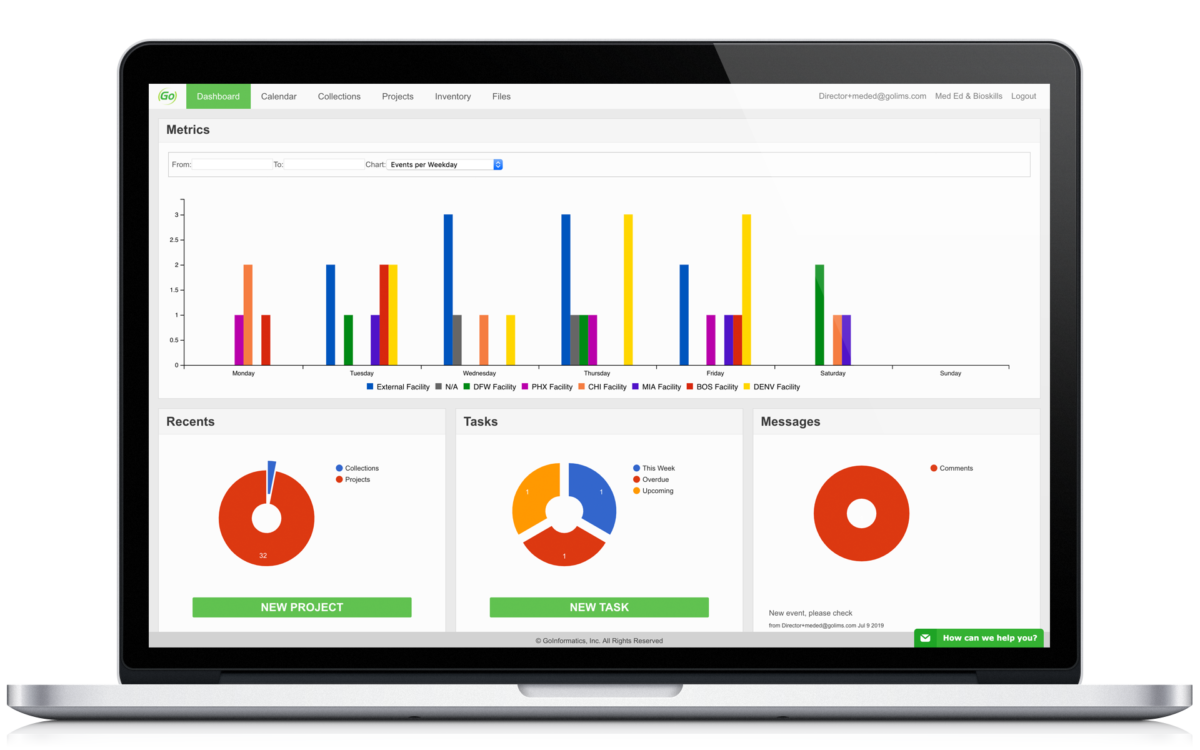
Centralize your research to make important data easily accessible and control who has access. Track key metrics, documents, and tasks.
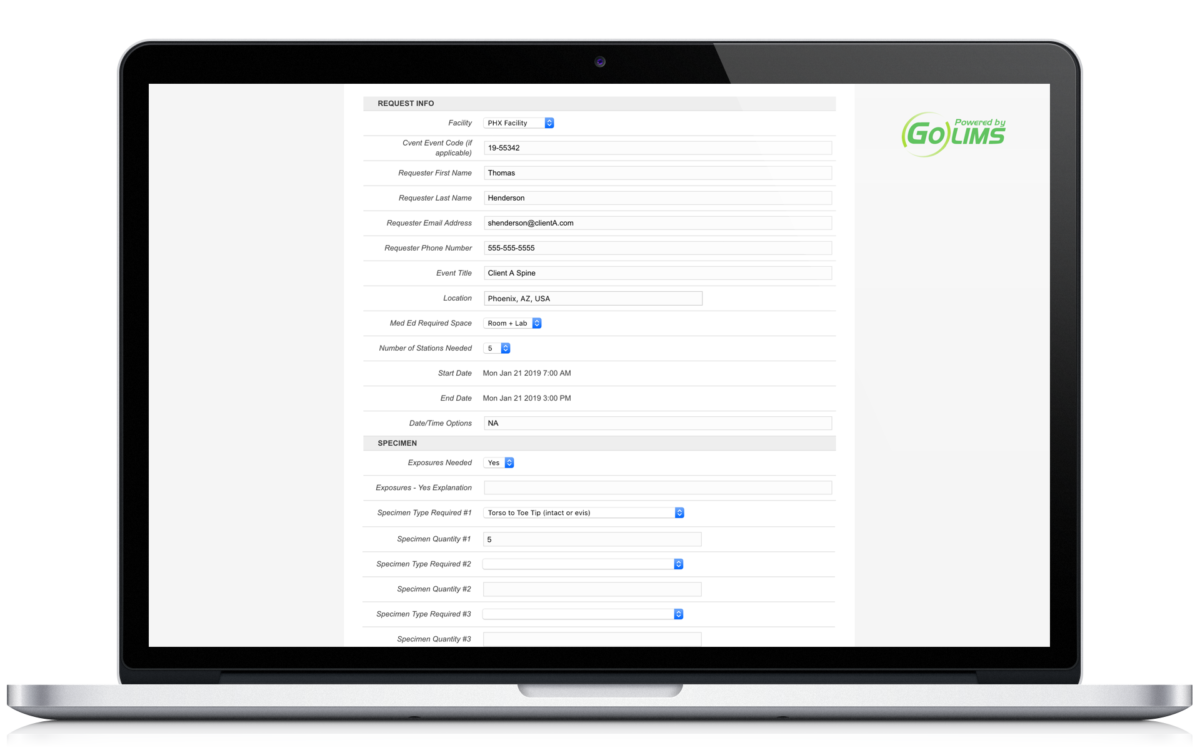
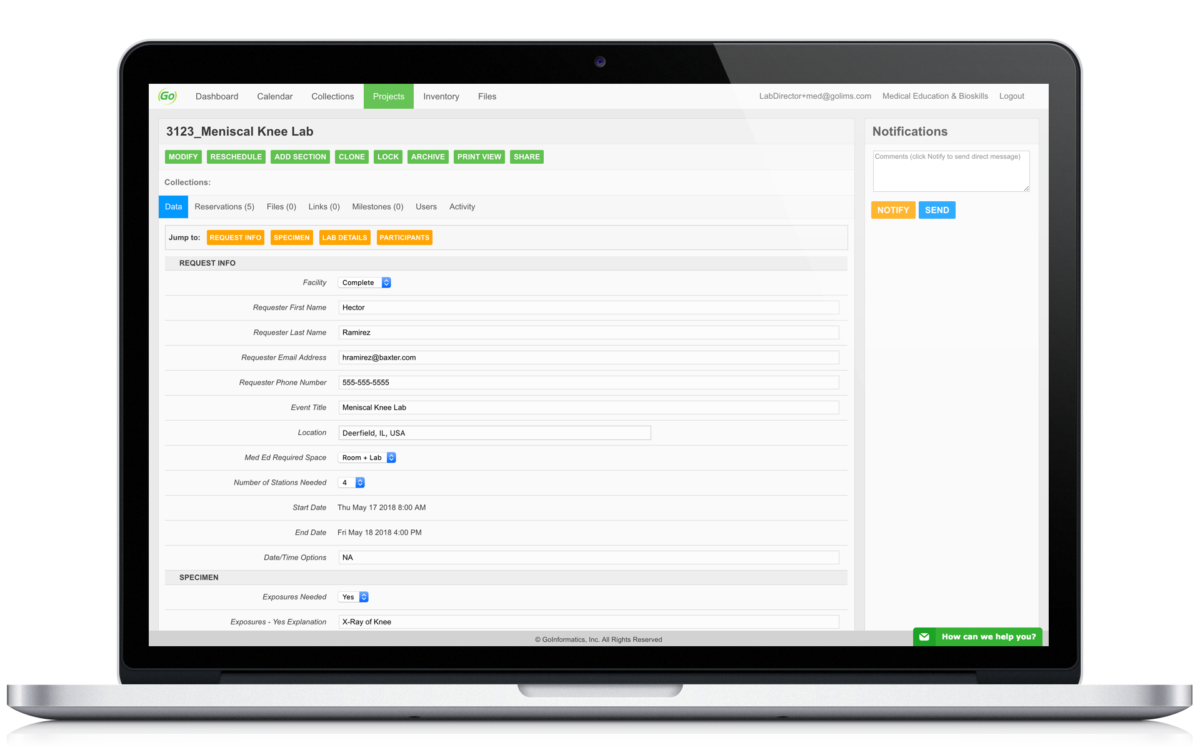
Contextually organize all related documents, regardless of file type. No more storing files in random folders not knowing their importance.
Insert graphs, images, and results information directly. Reference important data on a per user basis. Digitize your research information.
Communicate more efficiently by utilizing our in-app message and notification systems to keep all communication centralized and contextualized
Allow for the request and creation of new projects. Whether you receive requests from external collaborators or from internal team members, you can now standardize the intake of data.
All important data is collected and can be analyzed. Create custom administrative metrics to see how your team is performing and comparatively view this data.